Therefore it will undergo direct photolysis by sunlight. The proposed PEL was 2 ppm as an 8-hour TWA with a skin notation and the final rule establishes these limits.
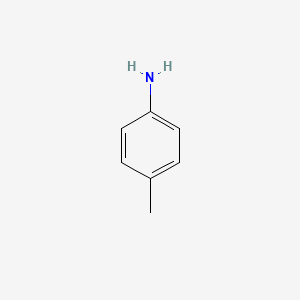
P Toluidine C6h4ch3nh2 Pubchem
They will not be considered in the grading.

. National Center for Biotechnology Information. The half-life for this reaction in air is estimated to be 19 hours. M-toluic acid is a methylbenzoic acid carrying a methyl substituent at position 3.
Do not include lone pairs in your answer. Decreasing order of basicity. Draw the structures of the major organic products you would expect from reaction of m-toluidine m-methylaniline with CH 3 I excess.
Has a floral-honey odor. Toluidines are aromatic amines used as building blocks for the synthesis of various chemicals. You do not have to consider stereochemistry.
The ACGIH has a 2-ppm 8-hour TWA with a skin notation. 4-Iodotoluene C7H7I CID 12207 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. It is a colorless liquid although commercial samples are often yellowish.
C 7 H 9 N. We review their content and use your feedback to keep the quality high. Answer 1 of 3.
O-Toluidine is a synthetic light sensitive light yellow liquid that is slightly soluble in water and miscible with carbon tetrachloride diethyl ether and ethanolThe hydrochloride is a synthetic light sensitive white crystalline powder that is soluble in dimethylsulfoxide and ethanol. You do not have to consider stereochemistry. Toluidine has been used in preparation of aromatic azo compounds and bidentate Schiff base ligands via condensation with salicylaldehyde.
The prefix of these three compounds indicate amino group positions relative to the methyl group on the same benzene ring. HPLC Analysis of Isomers of Toluidine on Core-Shell Mixed-Mode Coresep 100 Column. P toluidine o toluidine m toluidine aniline Basicity depends on the ability of electron donating groups that increases basicity of the lone pair of nitrogen.
It is the most important of the three isomeric toluidines. NTP 1992 CAMEO Chemicals. Draw the structures of the major organic products you would expect from reaction of m-toluidine m-methylaniline with HCl 1 equivalent.
You do not have to explicitly draw. C 7 H 8. Do not include lone pairs in your answer.
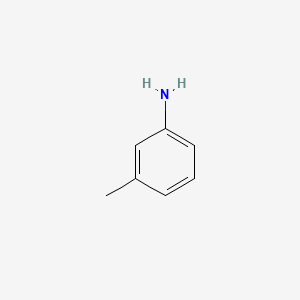
C 7 H 9 N. M-Toluidine is a light yellow liquid. Do not include lone pairs in your answer.
See the answer See the answer done loading. These isomers are o-toluidine m-toluidine and p-toluidine with the prefixed letter abbreviating respectively ortho. You do not have to explicitly draw H atoms.
M-toluic acid appears as white to yellowish crystals or mostly yellow flaky solid with some white flakes. Vapor-phase m-anisidine will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals. You do not have to explicitly draw H atoms.
Give the structures of the major organic products you would expect from reaction of m -toluidine m -methylaniline with the following reagents. 3-Amino-1-Methylbenzene 3-Methylaniline 3-Methylbenzenamine 3-Toluidine Meta-Toluidine 1-Aminophenyl Methane. Who are the experts.
Draw the structure of m-toluidine. Donating effect is most powerful on para then on ortho. Major metabolites are 4-amino-m-cresol and to a lesser extent N-acetyl-4-amino-m-cresol azoxytoluene o.
National Library of Medicine. You do not have to consider stereochemistry. See the answer See the answer done loading.
O-Toluidine ortho-toluidine is an organic compound with the chemical formula CH 3 C 6 H 4 NH 2. Draw the structures of the major organic products you would expect from the reaction of m-toluidine m-methylamiline with eqHNO_2 and H_2SO_4eq followed by. Show transcribed image text.
Draw the structure s of the major organic product s you would expect from reaction of m-toluidine m-methylaniline with HNO and HjSO followed by KCN and CuCN. This problem has been solved. A mathrmBr_2 1 equivalent b mathrmCH_3 I excess c mathrmCH_3 mathrmCOCl in pyridine d The product of c then mathrmHSO_3 mathrmCl.
Draw the structure s of the major organic product s you would expect from reaction of m-toluidine m-methylaniline with HCl 1 equivalent. M-Toluidine formerly had no OSHA permissible exposure limit. There are three structural isomers ortho-TOLUIDINE meta-TOLUIDINE and para-TOLUIDINE.
There are three isomers of toluidine which are organic compounds. They will not be considered in the grading. When m-toluidine was tested on the.
Toluidine is the other name of methylaniline or aminotoluene. M-toluidine - cas 108-44-1 synthesis structure density melting point boiling point. Draw the structure s of the major organic product s you would expect from reaction of m-toluidine m-methylaniline with HCl 1 equivalent.
It has a role as a human xenobiotic metabolite. You do not have to consider stereochemistry. M-Anisidine absorbs light at 285 nm.
They can be obtained from coal tar as by-products in the fractional distillation or can be prepared by. You do not have to explicitly draw H atoms. See the answer.
Aniline is lesser basic tha. O-Toluidine and o-toluidine hydrochloride are used primarily as intermediates in the manufacture of dyes. Experts are tested by Chegg as specialists in their subject area.
National Institutes of Health. All three are aryl amines whose chemical structures are similar to aniline except that a methyl group is substituted onto the benzene ring.

Answered Show How M Toluidine Can Be Converted Bartleby

M Toluidine Structure C7h9n Over 100 Million Chemical Compounds Mol Instincts

M Toluidine 99 Thermo Scientific Fisher Scientific

4 Methyl 2 Nitroaniline C7h8n2o2 Pubchem

Solved Draw The Structure Of M Toluidine Sn3 Hn Ch3 Chegg Com

Solved Draw The Structure S Of The Major Organic Product S Chegg Com
0 comments
Post a Comment